Of all the important elements of medical device new product development (NPD), project planning and management gets the least attention and is the most poorly performed. Full stop.
We expend tremendous product development effort to deliver patient safety, product performance, and physician ergonomics. We design for cost, manufacturability, and reliability. We agonize over the wording and acceptance criteria of every requirement. Our drawings are exquisitely toleranced. No spec is left unverified or unvalidated. Instruments and fixtures are IQ’d, OQ’d and PQ’d. Manufacturing processes are thoroughly validated. We justly take pride in these achievements. One medtech engineer told me that he sleeps better at night knowing his risk analysis was well-done.
Yet when it comes to planning a project, our attention wanders. We put in some effort, get frustrated by the complexity and ambiguity of the project tasks and dependencies, and call it “good enough.” We don’t put nearly the effort into planning that we put into FMEA’s or inspection instructions. We write something up, get it signed, and a couple of weeks later, project plans lie quietly buried and already outdated in a design history file. Most of the time, we don’t know how to make the plan any better, even if we wanted to.
Sound familiar? Software companies have shown us that there is a better way.
Continue reading “Top Five Reasons Why Tech and Software Companies Abandoned Traditional Project Planning” →
 Critical Action Planning is my attempt to combine key elements of Critical Chain planning with an Agile/Kanban philosophical approach, specifically for companies developing physical/hardware products. Like the Critical Chain, Critical Action project management is based on a detailed best-case task list for the complete project. Like Agile/Kanban, we don’t define task dependencies or projected task start or end dates. Also like Agile/Kanban, we estimate the amount of best-case work-units required to complete each task (e.g. in person days). Eliminating dependency and date planning dramatically simplifies the planning process, and makes the project plan parseable. Like Critical Chain, we add a buffer to the best-case plan, by including tasks to represent potential re-work or project iterations. We estimate work-units for these tasks too.
Critical Action Planning is my attempt to combine key elements of Critical Chain planning with an Agile/Kanban philosophical approach, specifically for companies developing physical/hardware products. Like the Critical Chain, Critical Action project management is based on a detailed best-case task list for the complete project. Like Agile/Kanban, we don’t define task dependencies or projected task start or end dates. Also like Agile/Kanban, we estimate the amount of best-case work-units required to complete each task (e.g. in person days). Eliminating dependency and date planning dramatically simplifies the planning process, and makes the project plan parseable. Like Critical Chain, we add a buffer to the best-case plan, by including tasks to represent potential re-work or project iterations. We estimate work-units for these tasks too. My colleague Jeff really opened my eyes when he introduced me to
My colleague Jeff really opened my eyes when he introduced me to  If your New Product Development (NPD) project management reliably delivers new products better, faster and cheaper than your competition, I’m impressed. Most of us are working hard to improve our NPD performance.
If your New Product Development (NPD) project management reliably delivers new products better, faster and cheaper than your competition, I’m impressed. Most of us are working hard to improve our NPD performance. The adoption of Agile project management techniques has been a key driver of improved new product development (NPD) productivity in tech and software companies (along with Moore’s law and industry adoption of technical standards). Here are ten ways Agile project management differs from traditional gantt-based management.
The adoption of Agile project management techniques has been a key driver of improved new product development (NPD) productivity in tech and software companies (along with Moore’s law and industry adoption of technical standards). Here are ten ways Agile project management differs from traditional gantt-based management.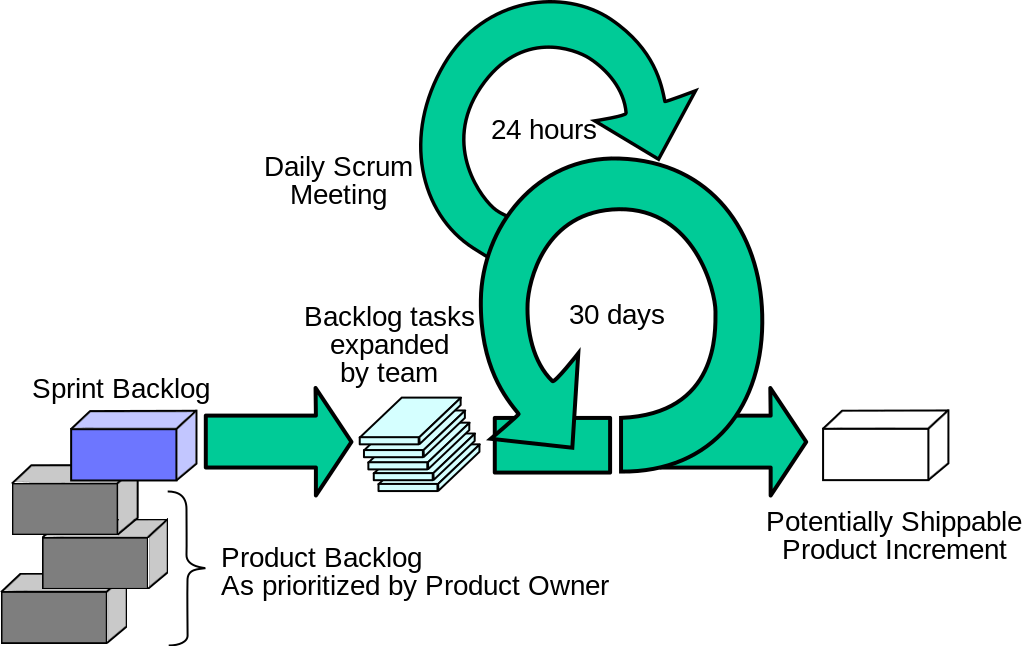 I’ve been writing recently about the wholesale abandonment of Gantt-based project management (including critical chain) by software and tech companies. In the software world, the Gantt approach has been wholly supplanted by Agile Project Management.
I’ve been writing recently about the wholesale abandonment of Gantt-based project management (including critical chain) by software and tech companies. In the software world, the Gantt approach has been wholly supplanted by Agile Project Management.